50MG
This product is for research purposes only. Not for human consumption.
Purity: >98% (HPLC verified)
Formulation: Powder
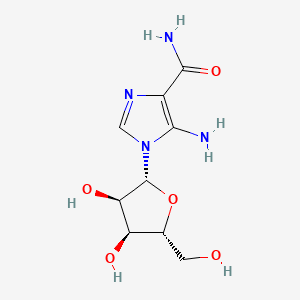
Molecular Formula: C9H15N4O8P
Molecular Weight: 338.21 g/mol
CAS Number: 3031-94-5
PubChem CID: 17513
AICAR
Overview
AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide), also known as acadesine or ZMP riboside, is a nucleoside analog that functions as a cell-permeable activator of AMP-activated protein kinase (AMPK), often called the master regulator of cellular energy metabolism. AICAR has gained significant attention in metabolic research and sports performance science for its remarkable ability to mimic many of the beneficial effects of exercise at the molecular level, earning it the designation of an exercise mimetic compound. When administered to cells or organisms, AICAR is taken up and phosphorylated intracellularly to form ZMP (AICAR monophosphate), which structurally and functionally mimics AMP (adenosine monophosphate), the natural activator of AMPK that accumulates during energy stress such as exercise, fasting, or hypoxia. Through this mechanism, AICAR tricks cells into behaving as though they are in an energy-depleted state even when ATP levels are actually normal, triggering the suite of adaptive metabolic responses that normally occur during physical activity. These responses include enhanced glucose uptake and utilization, increased fatty acid oxidation, stimulation of mitochondrial biogenesis, improved insulin sensitivity, and activation of catabolic pathways that generate ATP while suppressing anabolic processes that consume ATP. The compound has demonstrated impressive effects in animal models, including improved endurance performance, enhanced oxidative metabolism, increased slow-twitch muscle fiber content, and protection against metabolic diseases. However, the translation of these findings to human applications has been limited by safety concerns, particularly regarding potential cardiovascular effects and lymphoma risk identified in long-term animal studies. AICAR is prohibited by the World Anti-Doping Agency (WADA) for use in competitive sports due to its performance-enhancing potential, and several high-profile doping cases have involved athletes using AICAR. Despite its problematic safety profile for therapeutic or athletic use, AICAR remains an invaluable research tool for studying AMPK signaling, cellular metabolism, and the molecular mechanisms underlying exercise adaptation.
Mechanism of Action
AICAR exerts its metabolic effects through a well-characterized mechanism involving cellular uptake, intracellular phosphorylation, and activation of the AMPK signaling cascade. After administration, AICAR enters cells via equilibrative nucleoside transporters that normally transport adenosine and related nucleosides. Once inside cells, AICAR is rapidly phosphorylated by adenosine kinase to form ZMP, also called AICAR monophosphate. ZMP is an AMP analog that mimics the structure and function of AMP but is not efficiently converted to ATP, causing it to accumulate intracellularly. AMPK is a heterotrimeric serine/threonine kinase consisting of a catalytic α subunit and regulatory β and γ subunits, and it functions as a cellular energy sensor that is activated when the AMP:ATP ratio increases during energy stress. ZMP binds to the γ subunit of AMPK in the same manner as AMP, causing conformational changes that promote phosphorylation of the α subunit at Thr172 by upstream kinases, particularly LKB1 (liver kinase B1), and simultaneously inhibits dephosphorylation by protein phosphatases. This results in robust AMPK activation even in the presence of abundant cellular ATP. Activated AMPK then phosphorylates dozens of downstream target proteins involved in metabolism, gene transcription, and cellular homeostasis. Key metabolic effects include phosphorylation and activation of PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), the master regulator of mitochondrial biogenesis and oxidative metabolism, leading to increased mitochondrial number and function. AMPK phosphorylates and inactivates acetyl-CoA carboxylase (ACC), the enzyme that produces malonyl-CoA, a potent inhibitor of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme for fatty acid entry into mitochondria for oxidation. By reducing malonyl-CoA levels, AMPK activation enhances fat oxidation. AMPK promotes glucose uptake by stimulating translocation of GLUT4 glucose transporters to the cell surface in muscle and adipose tissue through an insulin-independent mechanism. It also activates glycolysis by phosphorylating and activating phosphofructokinase-2 (PFK-2). Furthermore, AMPK inhibits anabolic processes that consume ATP, including fatty acid synthesis, cholesterol synthesis, and protein synthesis (through inhibition of mTOR signaling), while promoting catabolic processes that generate ATP. At the transcriptional level, AMPK influences the expression of genes involved in metabolism through effects on transcription factors and coactivators. The net effect is a cellular metabolic reprogramming that resembles the adaptations induced by endurance exercise training, including enhanced oxidative capacity, increased fat utilization, improved glucose metabolism, and greater mitochondrial content and function. This exercise mimetic effect occurs without actual muscle contraction or the mechanical stress of physical activity, making AICAR a unique pharmacological tool for dissecting the molecular mechanisms of exercise adaptation.
Research Findings
Research into AICAR has spanned several decades and provided fundamental insights into AMPK signaling and metabolic regulation, while also revealing important limitations and safety concerns that have prevented its therapeutic application. Pioneering work by Narkar et al., published in Cell in 2008, demonstrated that AICAR administration to sedentary mice could reproduce many of the metabolic and performance adaptations normally seen only with exercise training. Treated mice showed increased expression of genes involved in oxidative metabolism, enhanced mitochondrial enzyme activity, increased proportion of slow-twitch oxidative muscle fibers, and most strikingly, a 44% improvement in treadmill running endurance compared to untreated controls - all without any exercise training. This landmark study demonstrated that pharmacological AMPK activation could partially mimic exercise adaptations at the molecular and functional levels, cementing AICAR status as an exercise mimetic. Subsequent research has explored AICAR effects on glucose metabolism and insulin sensitivity. Studies in insulin-resistant animal models, including obese rodents and models of type 2 diabetes, have shown that AICAR treatment enhances glucose uptake into skeletal muscle through insulin-independent mechanisms, improves whole-body glucose homeostasis, reduces hyperglycemia, and enhances insulin sensitivity. These effects occur through AMPK-mediated increases in GLUT4 translocation, enhanced glucose transporter expression, and improved mitochondrial function in metabolically compromised tissues. AICAR has also been shown to reduce hepatic glucose production, another key abnormality in diabetes, through AMPK effects on hepatic metabolism. Research into cardiovascular effects has yielded mixed findings. Some studies show protective effects of AICAR against ischemia-reperfusion injury in the heart, with AMPK activation promoting cardiac energy conservation and reducing injury during periods of reduced blood flow. However, other research has raised concerns about potential pro-arrhythmic effects and adverse cardiac remodeling with chronic AICAR exposure. Long-term animal toxicology studies have identified concerning safety signals, most notably an increased incidence of B-cell lymphomas in mice treated with high doses of AICAR for extended periods. While the mechanism and human relevance of this finding remain unclear, it represents a significant safety concern for any therapeutic application. Human studies with AICAR have been limited. Small clinical trials in patients with stable angina and ischemic heart disease have explored AICAR (given the name acadesine in clinical contexts) as a cardioprotective agent during cardiac surgery or ischemic events, with some studies showing potential benefits but others showing neutral or negative results. No large-scale trials have established clear clinical benefits, and development for cardiovascular indications has stalled. AICAR also gained notoriety in the sports doping world after WADA banned it in 2011 due to its performance-enhancing potential, and several professional cyclists and other athletes have been sanctioned for AICAR use. Despite the barriers to therapeutic use, AICAR remains widely used in research laboratories as a tool compound for activating AMPK and studying metabolic regulation, cellular energy sensing, and the molecular basis of exercise adaptation.
Research Applications
- AMPK pathway activation and signaling research
- Metabolic disease and diabetes treatment studies
- Exercise mimetic and endurance research
- Insulin resistance and glucose metabolism studies
- Mitochondrial biogenesis and function research
- Energy metabolism and cellular stress response studies
- Oxidative metabolism and fatty acid oxidation research
- Skeletal muscle adaptation and fiber type studies
- Cardiovascular metabolism and ischemic preconditioning research
- PGC-1α regulation and transcriptional control studies
- Anti-obesity and weight management research
- Sports performance enhancement research (prohibited by WADA)
Safety Profile
The safety profile of AICAR in humans is poorly characterized and raises significant concerns that have prevented its therapeutic development and led to its prohibition by the World Anti-Doping Agency (WADA). While AICAR has been administered to humans in limited clinical trials, these studies were small-scale, short-duration, and focused on specific clinical contexts (primarily cardiovascular applications), providing insufficient data to comprehensively assess safety particularly for the chronic administration that would be required for metabolic applications or performance enhancement. The primary safety concerns stem from animal toxicology studies that have revealed serious adverse effects with chronic or high-dose AICAR exposure. The most alarming finding is an increased incidence of B-cell lymphomas observed in mice treated with high doses of AICAR for extended periods. This carcinogenic signal, first identified in regulatory toxicology studies conducted for potential drug development, represents a critical safety red flag. While the mechanism underlying lymphoma development is not fully understood, it may relate to AICAR's effects on purine metabolism, cellular proliferation pathways, or immune function. The relevance of rodent lymphoma findings to human cancer risk is uncertain - not all rodent tumors translate to human cancer, and dose-response relationships, exposure duration, and species differences all influence extrapolation - but regulatory agencies and drug developers consider such findings seriously as potential indicators of human risk. The finding effectively halted therapeutic development of AICAR for chronic use indications despite its pharmacological activity. Cardiovascular safety concerns have also emerged from preclinical and limited human data. While some studies suggest AICAR may have cardioprotective effects under certain conditions (such as during ischemia), other research has indicated potential for adverse cardiac effects including arrhythmias, left ventricular hypertrophy with chronic administration, and possible promotion of pathological rather than physiological cardiac remodeling. The effects on cardiac function appear complex and potentially dose-, duration-, and context-dependent. Cardiovascular safety concerns are particularly relevant given that AMPK activation affects cardiac metabolism, contractility, and growth signaling, and dysregulated AMPK signaling has been implicated in certain cardiomyopathies. Clinical trials of acadesine (the clinical name for AICAR) in cardiac surgery and acute coronary syndromes have yielded mixed results, with some studies suggesting potential benefits but others showing neutral or concerning outcomes, and no clear therapeutic benefit has been established that would justify continued development despite the safety concerns. Metabolic effects require consideration - while many AICAR effects on glucose and lipid metabolism are beneficial in disease models, the compound's ability to activate AMPK independently of actual cellular energy status could theoretically cause metabolic imbalances or interfere with normal physiological energy sensing and homeostatic responses. Effects on hepatic function, renal function, and other organs have not been thoroughly characterized in humans. The limited human pharmacokinetic data available suggests AICAR has relatively short plasma half-life but variable tissue penetration and retention. Athletic use of AICAR for performance enhancement, while prohibited by WADA since 2011, has occurred despite the lack of human safety data and the concerning preclinical findings. Several high-profile doping cases have involved AICAR use by professional cyclists and other endurance athletes seeking the exercise mimetic benefits demonstrated in rodent studies. The actual efficacy of AICAR for enhancing human athletic performance remains uncertain given the lack of controlled human studies, but the willingness of some athletes to use a poorly characterized compound with serious safety signals illustrates the problematic interface between cutting-edge metabolic research and performance-seeking behavior. From a research use perspective, AICAR remains valuable as a laboratory tool compound for studying AMPK signaling and metabolic regulation in cells and animal models, where its utility outweighs safety concerns given appropriate experimental safeguards. However, for human therapeutic or performance-enhancement applications, the safety profile is inadequate and concerning. The combination of carcinogenic potential in animals, cardiovascular concerns, poorly characterized human pharmacology and toxicology, and absence of demonstrated clinical benefit in adequately powered trials make AICAR inappropriate for human use outside of carefully controlled clinical research settings with appropriate ethical oversight and informed consent. Individuals considering AICAR use should be aware of the serious safety uncertainties, the animal toxicology findings, the lack of human safety data, its prohibited status in sports, and the absence of quality control or purity guarantees for non-pharmaceutical sources.
Scientific References
Research Use Only
This product is intended for research purposes only and is not for human consumption, therapeutic use, or diagnostic applications. Please ensure compliance with all applicable regulations and institutional guidelines.