5MG
This product is for research purposes only. Not for human consumption.
Purity: >98% (HPLC verified)
Formulation: Lyophilized powder
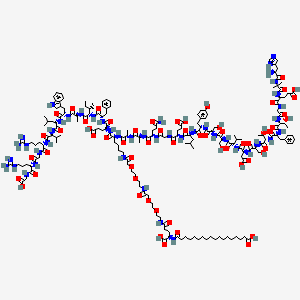
Molecular Formula: C187H291N45O59
Molecular Weight: 4113.64 g/mol
CAS Number: 910463-68-2
PubChem CID: 56843331
Semaglutide
Overview
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist that represents a breakthrough in metabolic medicine. As a synthetic peptide analog of human GLP-1, it mimics the action of this naturally occurring incretin hormone but with significantly enhanced stability and duration of action.
Originally developed for type 2 diabetes management, semaglutide has demonstrated remarkable efficacy across multiple therapeutic areas including weight management, cardiovascular protection, and metabolic health optimization.
The molecule features strategic modifications including an acylation side chain that enables albumin binding, resulting in a prolonged half-life of approximately one week, making it suitable for once-weekly administration. This pharmacokinetic profile has revolutionized treatment paradigms by improving patient compliance while maintaining consistent therapeutic effects.
Mechanism of Action
Semaglutide exerts its multifaceted effects through several interconnected pathways. Upon administration, it binds to and activates GLP-1 receptors distributed throughout the body, including pancreatic beta cells, the gastrointestinal tract, and specific regions of the central nervous system.
Pancreatic Effects
In pancreatic beta cells, GLP-1 receptor activation triggers glucose-dependent insulin secretion, meaning insulin release is enhanced only when blood glucose levels are elevated, thereby minimizing hypoglycemia risk. Simultaneously, semaglutide suppresses inappropriate glucagon secretion from pancreatic alpha cells, further contributing to improved glycemic control.
Gastrointestinal Effects
The peptide significantly delays gastric emptying through actions on the gastric smooth muscle and vagal afferents, which slows nutrient absorption and helps regulate postprandial glucose excursions.
Central Nervous System Effects
Perhaps most notably for weight management applications, semaglutide crosses the blood-brain barrier to act on GLP-1 receptors in the hypothalamus and brainstem, specifically targeting the arcuate nucleus and nucleus tractus solitarius. These central actions reduce appetite, enhance satiety, and decrease food cravings through modulation of pro-opiomelanocortin (POMC) neurons and other appetite-regulating pathways.
Cardiovascular Effects
Additionally, semaglutide has demonstrated cardioprotective effects independent of glucose lowering, possibly through anti-inflammatory mechanisms, improved endothelial function, and favorable effects on blood pressure and lipid metabolism.
Research Findings
The clinical research portfolio for semaglutide is exceptionally comprehensive, spanning multiple large-scale, international, randomized controlled trials.
STEP Clinical Trial Program
The landmark STEP (Semaglutide Treatment Effect in People with obesity) clinical trial program represents the most extensive investigation of semaglutide for weight management:
SUSTAIN Clinical Trial Series
Beyond the STEP program, the SUSTAIN clinical trial series established semaglutide's efficacy for type 2 diabetes management. SUSTAIN 6, a cardiovascular outcomes trial, revealed a 26% reduction in major adverse cardiovascular events (MACE) including cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. This cardiovascular benefit was observed across diverse patient populations and appears to extend beyond glucose control effects.
SELECT Trial
The SELECT trial, one of the largest cardiovascular outcomes trials in obesity medicine, enrolled over 17,000 participants with established cardiovascular disease and obesity or overweight (without diabetes) and demonstrated a 20% reduction in MACE with semaglutide 2.4 mg.
Additional Research Areas
Emerging research has also investigated semaglutide's potential benefits in:
Research Applications
- Type 2 diabetes management and glycemic control research
- Obesity treatment and long-term weight management studies
- Cardiovascular disease prevention and outcomes research
- Metabolic syndrome and insulin resistance studies
- Appetite regulation and satiety mechanism research
- Non-alcoholic fatty liver disease (NAFLD/NASH) investigations
- Cardiovascular risk reduction in obesity research
- Inflammation and oxidative stress studies
- Neuroprotection and cognitive function research
- Quality of life and obesity-related comorbidity studies
- Polycystic ovary syndrome (PCOS) research
- Bariatric surgery alternative studies
Safety Profile
Semaglutide has been extensively evaluated for safety across more than 10,000 participants in clinical trials, with exposure durations extending beyond 3 years in some studies. The safety profile is well-characterized and generally favorable when used according to prescribing guidelines.
Common Side Effects
The most commonly reported adverse events are gastrointestinal in nature:
- Nausea (approximately 20-44% of participants)
- Diarrhea (approximately 30%)
- Vomiting (approximately 24%)
These symptoms are typically mild to moderate in severity, tend to occur most frequently during dose escalation, and generally diminish over time as tolerance develops. Following a gradual dose titration schedule significantly reduces the incidence and severity of gastrointestinal side effects.
Serious Adverse Events
Serious adverse events are uncommon but have been documented and warrant appropriate monitoring:
Additional Safety Information
Hypoglycemia risk is minimal with semaglutide monotherapy due to its glucose-dependent mechanism of action, though risk increases when combined with insulin or sulfonylureas. Cardiovascular safety has been thoroughly evaluated, with trials demonstrating not only safety but also significant cardiovascular benefits. Renal safety data indicate that semaglutide is generally well-tolerated in patients with mild to moderate renal impairment.
Overall, when prescribed appropriately with proper patient selection, dose titration, and monitoring, semaglutide presents a favorable benefit-risk profile for its approved indications.
Scientific References
Research Use Only
This product is intended for research purposes only and is not for human consumption, therapeutic use, or diagnostic applications. Please ensure compliance with all applicable regulations and institutional guidelines.